 08:00 – 10:30
08:00 – 10:30 |
Session 1 - Morning |
|
 08:00 – 08:10
08:00 – 08:10 |
Opening Remarks Moderator Vivian Yam
|
|
 08:10 – 08:30
08:10 – 08:30 |
Keynote Lecture Rebecca Ruck, Merck Sharp & Dohme
Biocatalysis for the Synthesis of Pharmaceutical Agents at MSD
|
Biocatalysis for the Synthesis of Pharmaceutical Agents at MSD
Rebecca T. Ruck, MSD (Merck Sharp & Dohme). E-mail: rebecca_ruck@merck.com
The ideal process to prepare an API should be efficient, robust, cost-effective, green, and safe. In order to achieve these lofty goals on the timelines that are required to get important medicines to patients, we must innovate on the critical path. Such innovation often comes in the form of applying enabling technologies to complex synthetic and engineering challenges. This talk will focus on recent applications of novel capabilities that have allowed MSD to develop ideal processes across a diversity of APIs.
|
 08:30 – 08:50
08:30 – 08:50 |
Keynote Lecture Sarah Reisman, California Institute of Technology
Necessity is the Mother of Invention: Natural Products and the Chemistry They Inspire
|
Necessity is the Mother of Invention: Natural Products and the Chemistry They Inspire
Sarah E. Reisman, California Institute of Technology. E-mail: reisman@caltech.edu
The chemical synthesis of natural products provides an exciting platform from which to conduct fundamental research in chemistry and biology. Our group is currently pursuing the synthesis of a number of structurally complex natural products, with a particular focus on the development of new convergent fragment coupling strategies. The densely packed arrays of heteroatoms and stereogenic centers that constitute these polycyclic targets challenge the limits of current technology and inspire the development of new synthetic strategies and tactics. This seminar will describe the latest progress in our methodological and target-directed synthesis endeavors.
|
 08:50 – 09:05
08:50 – 09:05 |
Invited Lecture Yu Zhao, National University of Singapore
Catalytic Enantioselective Redox-Neutral Processes for Efficient Chemical Synthesis
|
Catalytic Enantioselective Redox-Neutral Processes for Efficient Chemical Synthesis
Yu Zhao, National University of Singapore. E-mail: zhaoyu@nus.edu.sg
The development of economical and selective catalytic methods is of significant importance for the promotion of sustainable chemical synthesis. My group at the National University of Singapore focuses on the identification of catalytic enantioselective redox-neutral transformations to access valuable chiral building blocks in organic synthesis. Such methods have the significant advantage of circumventing the redundant oxidation/reduction steps to reduce waste production in chemical synthesis. In particular, the stereoconvergent chiral amine synthesis from readily available racemic alcohols via borrowing hydrogen and enantioselective isomerization of racemic alkenyl alcohols will be discussed.
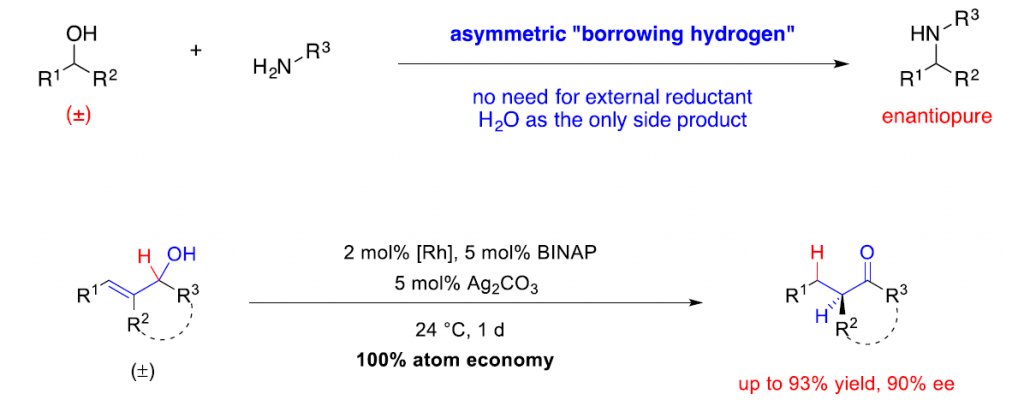
References
(1) Zhang, Y.; Lim, C.-S.; Sim, D. S. B.; Pan, H.-J.; Zhao, Y. Angew. Chem. Int. Ed. 2014, 53, 1399–1403.
(2) Rong, Z.-Q.; Zhang, Y.; Chua, H. B. R.; Pan, H.-J. Zhao, Y. J. Am. Chem. Soc. 2015, 137, 4944–4947.
(3) Pan, H.-J.; Zhang, Y.; Shan, C.; Yu, Z.; Lan, Y.*; Zhao, Y.* Angew. Chem. Int. Ed. 2016, 55, 9615-9619.
(4) Lim, C.S.; Quach, T. T.; Zhao, Y. Angew. Chem. Int. Ed. 2017, 56, 7176-7180.
(5) Xu, G.; Yang, G.; Wang, Y.; Shao, P.-L.; Yau, J. N. N.; Liu, B.; Zhao, Y.; Sun, Y.; Xie, X.; Wang, S.; Zhang, Y.; Xia, L.; Zhao, Y. Angew. Chem. Int. Ed. 2019, in press.
(6) Liu, T.-L.; Ng, T. W.; Zhao, Y. J. Am. Chem. Soc. 2017, 139, 3643-3646.
(7) Huang, R.-Z.; Lau, K. K.; Li, Z.; Liu, T.-L.; Zhao, Y. J. Am. Chem. Soc. 2018, 140, 14647–14654.
|
 09:05 – 09:10
09:05 – 09:10 |
Interactive Break
|
|
 09:10 – 09:30
09:10 – 09:30 |
Keynote Lecture Mark Thompson, University of Southern California
Modern Alchemy: Making Coinage Metals Act Like Iridium
|
Modern Alchemy: Making Coinage Metals Act Like Iridium
Mark Thompson, Department of Chemistry, University of Southern California. E-mail: met@usc.edu
Heavy-metal containing phosphors, especially iridium-based emitters, have become the standard in high performance mobile displays and televisions. The high-spin orbit coupling in these compounds facilitates the efficient harvesting of both singlet and triplet excitons generated in the electroluminescent process. An alternative to iridium-based emitters are solely-organic emitters based on Thermally Assisted Delayed Fluorescence (TADF). Heavy-metal and TADF emitters give similar OLED performance, which stems from the fact that they give very similar radiative lifetimes. We have found that the key to achieving higher performance for TADF emitters is to put the metal ions back into the TADF emitters. My talk will focus on the photophysical and electroluminescent properties of two-coordinate copper, silver and gold carbene complexes, i.e. (carbene)MI(donor), where the donor is an amide or aryl group. These complexes show high phosphorescence quantum yield (ΦPL = 0.7 – 1.0), with radiative lifetimes in 0.4-3 μs regime. Cryogenic photophysical measurements show these TADF emitters have singlet-triplet gaps as low as 150 cm-1 (20 meV). We have prepared organic LEDs with these dopants and achieved >20% EQE for green emissive OLEDs and > 12% for blue emissive OLEDs, both at comparatively low drive voltages.
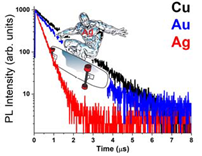
|
 09:30 – 09:45
09:30 – 09:45 |
Invited Lecture Gong Chen, Nankai University
C-H Functionalization Strategy for Synthesis of Complex Peptides and Carbohydrates
|
C-H Functionalization Strategy for Synthesis of Complex Peptides and Carbohydrates
Gong Chen, State Key Laboratory of Elemento-organic Chemistry, Nankai University. E-mail: gongchen@nankai.edu.cn
We will discuss our recent investigation of palladium-catalyzed bidentate auxiliary-directed C−H functionalization reactions for αAA substrates. Our strategies utilize two different types of amide-linked auxiliary groups, attached at the N or C terminus of αAA substrates, to exert complimentary regio- and stereo-control on C−H functionalization reactions through palladacycle intermediates. These strategies have been applied to the total synthesis of complex peptide natural products. We will then discuss efficient and generally applicable strategies for constructing cyclophane-braced peptide macrocycles using palladium-catalyzed intramolecular C(sp3)-H arylation reactions. Latest advance in Pd-catalyzed C-H glycosylation for synthesis of complex C-aryl glycosides will also be discussed.
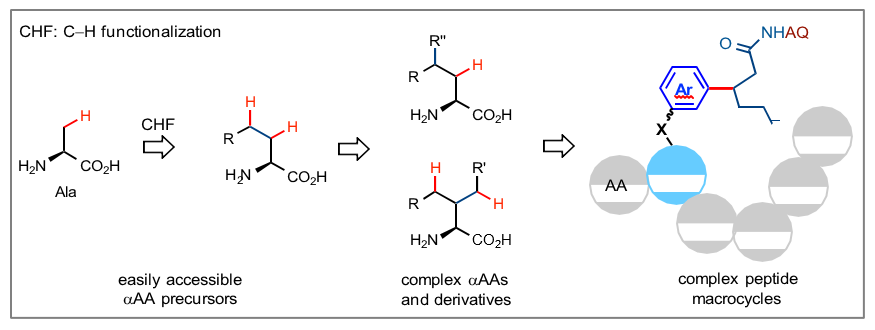
References
[1] He, G. ; Wang, B.; Nack, W.; Chen, G. Acc. Chem. Res. 2016, 49, 635.
[2] Wang, B.; Liu, Y.; Jiao, R.; Feng, Y.; Li, Q.; Chen, C.; Liu, L.; He, G.; Chen, G. J. Am. Chem. Soc. 2016, 138, 3926.
[3] Li, B.; Li, X.; Han, B.; Chen, Z.; Zhang, X.; He, G.; Chen, G.* J. Am. Chem. Soc. 2019, 141, 9401-9407.
|
 09:45 – 10:00
09:45 – 10:00 |
Invited Lecture Yunho Lee, Seoul National University Metal-Ligand Cooperative Transformations of Small Molecules
|
Metal-Ligand Cooperative Transformations of Small Molecules
Yunho Lee, Seoul National University, Email: yunhochem@snu.ac.kr
Metal-ligand cooperation (MLC) is currently receiving much attention as a novel synthetic methodology to expand the role of transition metals in organometallic reactions. The ligand in MLC delivers necessary electron, proton and/or a functional group to assist metal. While noninnocent phosphine ligands are relatively rare, our group recently reported a unique MLC by employing an anionic PPP ligand (PPP– = –P[2-PiPr2-C6H4]2). In this system, the reversible phosphide/phosphinite interconversion of a PPP ligand is coupled with a 2-electron redox change of nickel. Interestingly, the central phosphide moiety can act as a single electron donor, which was recognized from the studies of analogous nickel amide species. In this presentation, the MLC redox chemistry of a (PPP)M scaffold (M = Ni or Co) and its application in small molecule transformation will be presented. Both nickel and cobalt complexes reveal the reversible formation of a P–P bond involving a single electron exchange between metal and P. A P–P bond containing dimer, (P2P-PP2){Ni(CO)}2, shows radical-type reactivity toward various substrates under visible-light conditions. Detection of a metal-phosphinyl radical species formed upon irradiation was established by EPR spectroscopy and through experiments employing radical scavengers. The reactions with a phenoxyl radical clearly show the difference in reactivity of close-shell nickel and open-shell cobalt species.
- Kim, S., Kim, Y.-E., Lee, Y., Top. Organomet. Chem. 2021, 68, 71-94.
- Kim, S. and Lee, Y.* Inorg. Chem. Front. 2020, 7, 1172-1181.
- Kim, Y.-E. and Lee, Y.* Angew. Chem. Int. Ed. 2018, 57, 14159-14163.
- Kim, Y.-E., Kim, O., Oh, S., Kim, S., Kim, J., Han, S. W. and Lee, Y.* J, Am. Chem. Soc. 2015, 137, 4280-4283.
|
 10:00 – 10:30
10:00 – 10:30 |
Plenary Lecture Carolyn R. Bertozzi, Stanford University, USA Therapeutic Opportunities in Glycoscience
|
Therapeutic Opportunities in Glycoscience
Carolyn R. Bertozzi, Anne T. and Robert M. Bass Professor of Chemistry and Professor of Chemical & Systems Biology and Radiology (by courtesy), Stanford University; Baker Family Director, Stanford ChEM-H; Howard Hughes Medical Institute. E-mail: bertozzi@stanford.edu
Cell surface glycans constitute a rich biomolecular dataset that drives both normal and pathological processes. Their “readers” are glycan-binding receptors that can engage in cell-cell interactions and cell signaling. Our research focuses on mechanistic studies of glycan/receptor biology and applications of this knowledge to new therapeutic strategies. Our recent efforts center on pathogenic glycans in the tumor microenvironment and new therapeutic modalities based on the concept of targeted degradation.
|
 11:00 – 13:00
11:00 – 13:00 |
Live Poster Q&A Sci-Meetings
|
|
 14:00 – 16:20
14:00 – 16:20 |
Session 2 - Evening |
|
 14:00 – 14:05
14:00 – 14:05 |
Opening Remarks Moderator Kai Rossen
|
|
 14:05 – 14:25
14:05 – 14:25 |
Keynote Lecture Yan-Mei Li, Tsinghua University
Chemical Synthetic Glycopeptide Vaccines for Cancer Immunotherapy
|
Chemical Synthetic Glycopeptide Vaccines for Cancer Immunotherapy
Yan-Mei Li, Tsinghua University. E-mail: liym@mail.tsinghua.edu.cn
Compared with traditional approaches, cancer immunotherapy is considered a safer and more effective way for cancer treatment. Vaccines play an important role in immunotherapy, with the carbohydrates and glycopeptides that are aberrantly expressed on tumor cell surfaces acting as promising antigens for the development of tumor vaccines. To avoid the side effects of the carrier proteins used to improve the immunogenicity of these antigens, fully synthetic multicomponent vaccines that contain B-cell epitopes, T-helper cell epitopes, and immunostimulants were developed.
In our study, MUC1 glycopeptides were synthesized as the target antigens for cancer vaccines. And to improve the immunogenicity of MUC1 glycopeptides, we introduced Bovine Serum Albumin as the carrier protein at first, then replaced it by T-cell epitopes to reduce the side effects in our later work. To enhance immune response, the adjuvant Pam3CSK4, agonist of iNKT cell and the STING agonist were constructed in our three-component vaccine. Inspired by subject-object chemistry, self-assembly vaccines containing Q11 peptide and B-cell epitopes, Pam3CSK4 and T21-MUC1 were developed. Also, a novel DNA supramolecular hydrogel based vaccine was carried out. These vaccines we developed can efficiently trigger the immune system to elicit a high level of antibodies against MUC1 glycopeptides.
|
 14:25 – 14:40
14:25 – 14:40 |
Invited Lecture Guangyu Zhu, City University of Hong Kong
Development of Pt(IV) Anticancer Prodrugs Controllably Activated by Visible Light
|
Development of Pt(IV) Anticancer Prodrugs Controllably Activated by Visible Light
Guangyu Zhu, City University of Hong Kong. E-mail: guangzhu@cityu.edu.hk
Despite the broad clinical applications of platinum-based anticancer drugs, including cisplatin, their side-effects and resistance issues have encouraged researchers to look for novel metal-based anticancer complexes. Nontraditional platinum compounds, especially Pt(IV) complexes, have been extensively studied and hold great promise to be further developed as the next-generation platinum drugs.[1] Selective activation of prodrugs within a tumor is particularly attractive because of their low damage to normal tissue. In this presentation, I will introduce the design, photoactivation mechanism, and antitumor activity of visible light-activatable Pt(IV) prodrugs.[2,3] These small-molecule prodrugs have controllable activation properties: They are shown to be inert in the dark, but under short-period irradiation with low-intensity visible light, without the need of any external catalyst, the prodrugs are rapidly reduced. The prodrugs display superior antitumor activity both in vitro and in vivo in human carcinoma models. The controllable activation property and superior antitumor activity of these prodrugs may suggest a novel strategy for the design of visible-light-activatable platinum prodrugs to reduce the adverse effects and conquer drug resistance of traditional platinum chemotherapy.
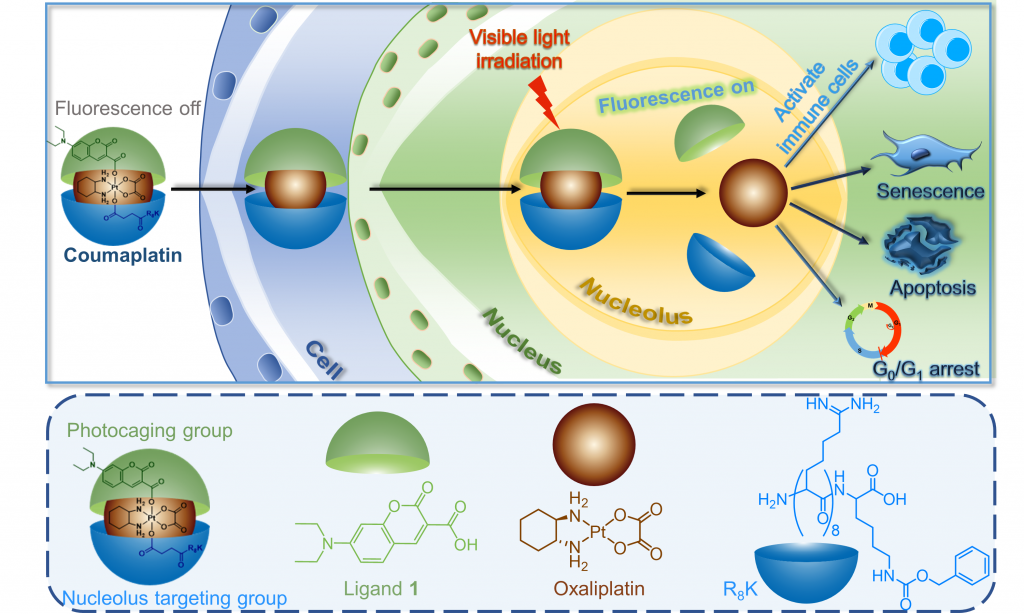
References
- Wang Z., Deng Z., Zhu, G, Dalton Trans 2019, 48, 2536-2544 [Invited Review and Cover Article]
- Wang Z., Zhu, G, et al. Chem 2019, 5, 3151-3165
- Deng Z., Zhu, G., et al. J Am Chem Soc 2020, 142, 7803-7812 [Supplementary Cover]
|
 14:40 – 14:55
14:40 – 14:55 |
Invited Lecture Liang Deng, Shanghai Institute of Organic Chemistry
Low-Coordinate Low-Valent 3d Metal Complexes with NHC and Alkene Ligation
|
Low-Coordinate Low-Valent 3d Metal Complexes with NHC and Alkene Ligation
Liang Deng, Shanghai Institute of Organic Chemistry, CAS. E-mail: deng@sioc.ac.cn
The knowledge on the formation, structure, and reactivity of low-coordinate 3d metal species forms the basis for the development of new metal-catalyzed organic transformations and also disclosing the mysterious mechanisms of enzymatic catalysis and “single-atom” catalysis. Aiming to deepen our knowledge on this type of reactive metal species, we have been working on the chemistry of low-coordinate low-valent cobalt, iron, and manganese complexes with N-heterocyclic carbene (NHC) and olefin ligation for years. This ligand set is found effective in stabilizing three-coordinate cobalt(0,-I), iron(0,-I), and manganese(0) complexes in the forms of [(NHC)M(olefin)2]n (n = 0, 1-) (Figure 1). In this presentation, the synthesis and electronic structure of the three- coordinate low- valent metal complexes, as well as their reactivity toward alkenes, alkynes, C-nitroso compounds, hydrosilanes, and hydrophosphines will be discussed.
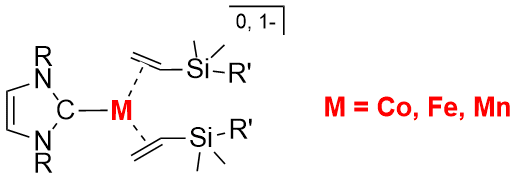 Figure 1 |
 14:55 – 15:15
14:55 – 15:15 |
Keynote Lecture Eiichi Nakamura, University of Tokyo
Capturing the Moment of Reaction Events – "Visual Molecular Science" Enabled by Atomic Resolution Electron Microscopy
|
Capturing the Moment of Reaction Events – “Visual Molecular Science” Enabled by Atomic Resolution Electron Microscopy
Eiichi Nakamura, The University of Tokyo. E-mail: nakamura@chem.s.u-tokyo.ac.jp
Public screening of Lumière brothers’ short films in 1895 created a big sensation, because moving images carry overwhelmingly richer information than still images. Moving images have never been popular in chemistry: Chemists may have been largely satisfied by chemical formulae and static molecular images, probably because they are so well trained that they understand chemistry only with static images. This situation is changing. We reported in 2007 the first motion pictures of the conformational change of a saturated hydrocarbon molecule in a carbon nanotube, for which we coined a word, SMART-EM (single molecule atomic resolution real time electron microscopy).1 Most recently, we reported the whole process of crystal nucleation and growth of a NaCl crystal using an atomic resolution transmission microscope equipped with a camera capable of capturing more than 1000 frames per second.2 The results shed light on the atomistic details on how a crystal forms from the scratch – the SMART-EM research is stepping into the next stage after a run-up period.
- E. Nakamura, Acc. Chem. Res., 50, 1281–1292 (2017).
- T. Nakamuro, M. Sakakibara, H. Nada, K. Harano, E. Nakamura, J. Am. Chem. Soc., 143, 1763-1767 (2021).
|
 15:15 – 15:20
15:15 – 15:20 |
Interactive Break
|
|
 15:20 – 15:40
15:20 – 15:40 |
Keynote Lecture Frank Würthner, Universität Würzburg
Functional Dyes for Organic Electronics and Photonics
|
Functional Dyes for Organic Electronics and Photonics
Frank Würthner, Universität Würzburg, Institut für Organische Chemie & Center for Nanosystems Chemistry, 97074 Würzburg, Germany. Email: wuerthner@uni-wuerzburg.de
Despite the fact that many functional properties of π-conjugated scaffolds only originate by the substitution of aromatic hydrocarbons with electron-donating and -withdrawing groups, research on larger π-scaffolds has so far been focused on the pristine subunits of graphene, carbon nanotubes or other polycyclic aromatic hydrocarbons. In contrast, driven by our ongoing interest in functional dyes and n-channel organic semiconductors, we recently focused our attention on planar and bowl-shaped polycyclic aromatic hydrocarbons with low-lying LUMO levels that are realized by the incorporation of boron centers or functionalization with multiple dicarboximide units in the π -scaffold’s periphery. Due to a lack of available synthetic methodologies towards such desirable molecules we developed a new cross-coupling- annulation cascade reaction for the synthesis of large-sized planar and contorted electron-poor π -scaffolds. In this talk I will discuss our new synthetic methodology and give first insights into the supramolecular and functional properties for a new class of nanosized functional π- conjugated molecules.
|
 15:40 – 15:55
15:40 – 15:55 |
Invited Lecture Mi Hee Lim, Korea Advanced Institute of Science & Technology
Bioinorganic Strategies to Study Multiple Facets in Dementia
|
Bioinorganic Strategies to Study Multiple Facets in Dementia
Mi Hee Lim, Korea Advanced Institute of Science & Technology (KAIST). E-mail: miheelim@kaist.ac.kr
Alzheimer’s disease (AD), associated with degeneration of neurons and synapses in the brain, leads to motor impairment and eventual fatality. Neurodegeneration is related to interconnected features, including amyloid-β (Aβ) aggregate deposition, metal ion dyshomeostasis and miscompartmentalization, and inflammation and increased oxidative stress induced by overproducing reactive oxygen species (ROS). The interrelations between some of these pathological factors have been investigated. Metal ions are found in the Aβ plaque and contribute to Aβ-related toxicity and oxidative stress. ROS have been shown to increase Aβ aggregate generation. Our understanding of the correlation between these elements and AD pathology has been very limited, however. There is currently no cure for AD. To identify an effective cure, we require a better understanding of the relationship between various causative factors of this disease. Toward this goal, we need suitable chemical tools capable of targeting and regulating its multiple underlying factors simultaneously. In this presentation, the rational design and preparation of our chemical tools will be discussed with detailed molecular-level investigations of their interactions and reactivities with targets in vitro as well as their efficacies in vivo.
|
 15:55 – 16:15
15:55 – 16:15 |
Keynote Lecture Fabrice Gallou, Novartis Pharma AG
Sustainability as a Trigger for Innovation
|
Sustainability as a Trigger for Innovation
Fabrice Gallou. Chemical & Analytical Development, Novartis Pharma AG, 4056 Basel, Switzerland. E-mail: fabrice.gallou@novartis.com
During our evaluation of the potential of surfactant technology in collaboration with academic collaborators Professors Lipshutz and Handa,(1,2,3) we have identified a variety of straightforward and highly advantageous transformations and then screened and applied them successfully on-scale.(4) Implementation of the technology typically results in significant benefits across our entire portfolio, not just from an environmental standpoint, but also from an economic and productivity perspective. To name a few: reduction of organic solvent consumption, water use, and cycle time; milder reaction conditions; and improved yields and selectivities, which all contribute to improved process performance and lower manufacturing costs.(5)
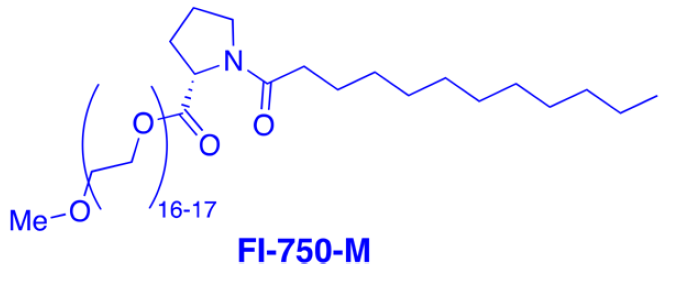 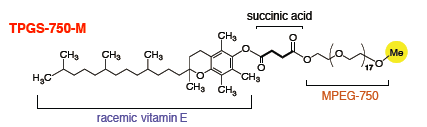
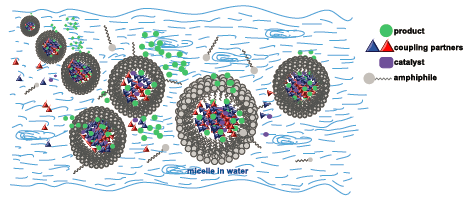 Modern non-ionic surfactants for micellar catalysis in water. These surfactant-mediated reactions can be up-scaled in the already existing multipurpose facilities of pharmaceutical or chemical organizations, using a catalytic amount of a combination of a nonionic designer surfactant (e.g. TPGS-750-M, FI-750-M) in water, and a well-chosen organic cosolvent instead of traditional and undesirable organic solvents.(6) We now start gaining insight into the physical phenomena involved and the role of the various components of the system and utilize this know-how to design even better catalytic systems.(7) Recent applications on other challenging catalytic transformations,(8) biocatalysis,(9) photoredox,(10) or flow transformations (11) have or will be reported shortly.
[1] See for example: Science 2015, 349, 1087; Ang. Chem. Int. Ed. 2016, 55, 8979; Ang. Chem. Int. Ed. 2016, 55, 4914.
[2] J. Am. Chem. Soc. 2013, 135, 17707; Org. Lett. 2015, 17, 4734; Org. Lett. 2015, 17, 3968; Org. Proc. Res. Dev. 2016, 20, 1104; Nat. Rev. Chem. 2018, 2, 306.
[3] Chem. Sci. 2017, 8, 6354; ACS Catal. 2018, 7, 10, 7245..
[4] Green Chem. 2016, 18, 14; Org. Proc. Res. Dev. 2018, 22, 1453.
[5] ACS Sustain Chem. Eng. 2016, 4, 5838.
[6] Org. Proc. Res. Dev. 2016, 20, 1388.
[7] Green Chem. 2018, 20, 3436; ACS Catal. 2017, 7, 10, 7245; Org. Lett. 2017, 19, 194; Eur. J. Org. Chem. 2018, 24, 6778; Org. Lett. 2018, 20, 2902; Chem. Sci.2019, accepted; ACS Cat 2019, accepted DOI: 10.1021/acscatal.9b02316.
[8] ACS Catal. 2019, 9, 3, 2423; Helv. Chim. Acta, 2019, 102, e1900024; ACS Catal. 2019, 9, 2423; Chem. Sci. 2019, 10, 3481.
[9] Nat. Comm. 2019, 10, 2169.
[10] Nat. Comm. 2019, 10, 1837 ; Green Chem. 2018, 20, 1233; Green Chem. 2018, 20, 1784; J. Org. Chem.2018, 83, 7366.
[11] unpublished results.
|
 16:15 – 16:20
16:15 – 16:20 |
Closing Remarks - Day 1 |
|
 08:00 – 10:35
08:00 – 10:35 |
Session 3 - Morning |
|
 08:00 – 08:05
08:00 – 08:05 |
Opening Remarks Moderator Vivian Yam
|
|
 08:05 – 08:35
08:05 – 08:35 |
Plenary Lecture Paul Chirik, Princeton University
Reimagining the Periodic Table: Addressing Sustainability Challenges in the 21st Century Through Catalysis
|
Reimagining the Periodic Table: Addressing Sustainability Challenges in the 21st Century Through Catalysis
Paul J. Chirik, Department of Chemistry, Princeton University, Princeton, NJ 08544 USA. Email: pchirik@princeton.edu
How chemists interact with and ultimately use the elements on the Periodic Table is one of the primary sustainability challenges for the 21st century. Applications ranging from alternative energy to catalysis need to deploy Earth-abundant elements such as iron rather than terrestrially rare ones such as rhodium that have a large environmental footprint associated with mining and purification. Our research group is exploring the new chemistry enabled by catalysis with a range of Earth-abundant transition metals. At the core of this challenge is how electrons flow in chemical reactions as compared to precious metals. By using a combination of redox-active ligands, those that undergo reversible electron transfer with a transition metal, or strong-field chelate ligands to surpress deleterious radical chemistry, unique transformations have been identified. The strong-field approach has found application in the asymmetric hydrogenation of alkenes with activity, enantioselectivity, and a solvent profile (methanol vs. dichloromethane) superior to state-of-the-art rhodium catalysts. This approach has also been applied to the C(sp2)-H borylation of alkenes with unique site selectivity that is distinct from precious metals and opens new, streamlined pathways to active pharmaceutical ingredients. The weak-field approach employing redox-active ligands has opened new cycloaddition pathways for the upgrading of abundant hydrocarbons. Using tridentate ligands, selective [2+2] alkene-alkene cycloadditions to cyclobutanes has been realized, and with bidentate chelates a [4+4] pathway has been discovered. My lecture will focus on the development and discovery of these catalysts and their impact on contemporary sustainability challenges.
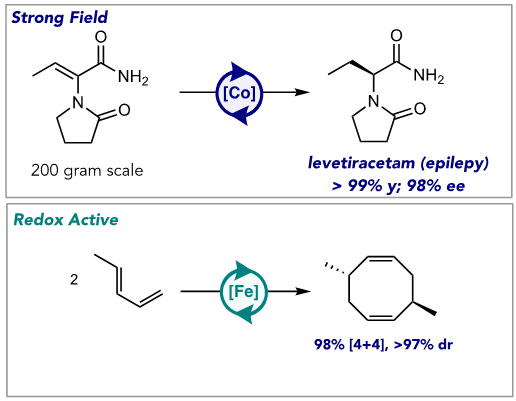 Figure 1. Chemistry enabled by catalysis with Earth-abundant transition metals. Representative Publications:
- Kim, S.; Zhong, H.; Park, Y.; Loose, F.; Chirik, P. J. “Catalytic hydrogenation of a manganese(V) nitride to ammonia.” J. Am. Chem. Soc. 2020, 142, 9518-9524.
- Joannou, M. V.; Hoyt, J. M.; Chirik, P. J. “Investigations into the mechanism of inter- and intramolecular iron-catalyzed [2+2] cycloaddition of alkenes.” J. Am. Chem. Soc. 2020, 142, 5314-5330.
- Viereck, P.; Krautwald, S.; Pabst, T. P.; Chirik, P.J. “A boron activating effect enables cobalt-catalyzed asymmetric hydrogenation of sterically hindered alkenes.” J. Am. Chem. Soc. 2020, 142, 3923-3930.
- Friedfeld, M. R.; Zhong, H.; Ruck, R. T.; Shevlin, M.; Chirik, P. J. “Cobalt-catalyzed asymmetric hydrogenation of enamides enabled by single-electron reduction.” Science 2018, 360, 888-893.
- Pabst, T. P.; Obligacion, J. V.; Rochette, E.; Pappas, I.; Chirik, P. J. “Cobalt-catalyzed borylation of fluorinated arenes: Thermodynamic control of C(sp2)–H oxidative addition results in ortho-to-fluorine selectivity.” J. Am. Chem. Soc. 2019, 141, 15378-15389.
- Bezdek, M.; Guo, S.; Chirik, P. J. “Coordination induced bond weakening of ammonia, water, and hydrazine with a molybdenum complex.” Science 2016, 354, 730-733.
|
 08:35 – 08:55
08:35 – 08:55 |
Keynote Lecture Hong-Cai Zhou, Texas A&M University
|
|
 08:55 – 09:10
08:55 – 09:10 |
Invited Lecture Qiuling Song, Huaqiao University
Metallate Shift Enabled by Tetracoordinate Boron Intermediates
|
Metallate Shift Enabled by Tetracoordinate Boron Intermediates
Qiuling Song, Institute of Next Generation Matter Transformation, College of Material Sciences Engineering, Huaqiao University and College of Chemistry, Fuzhou University. E-mail: qsong@hqu.edu.cn
Organoboron compounds have been widely recognized as versatile intermediates in organic synthesis due to their unique stability toward both air and moisture, operational simplicity, non-toxicity and properties prone to the construction of new C-C or C-heteroatom bonds.1 The transformation of organoboron compounds is usually carried out through tetracoordinate boron intermediates, mainly including additions to unsaturated bonds, rearrangements, transmetallations and so on. Despite the great progress, there are still shortcomings on tetracoordinate boron intermediates, and the transformative strategies of them are restricted. The rearrangement reactions of tetracoordinate boron intermediates are very common, however, they are mainly 1,2-metallate shift, long-distance (over three atoms) or multiple metallate migrations are rare. We designed and developed several novel strategies involving tetracoordinate boron intermediates to construct C-C bonds through long-distance or multiple migration reactions (Scheme 1). 2
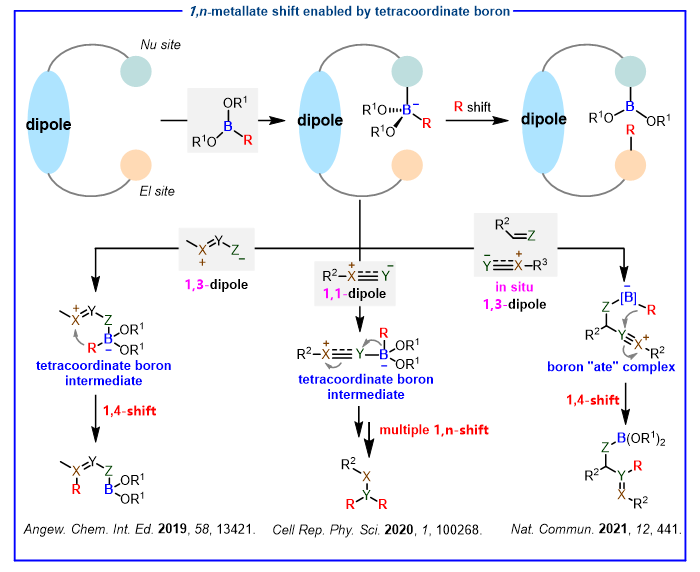 Scheme 1. Our design and tetracoordinate boron intermediate enabled 1,n-metallate shift References
- For an example of review: D. G. Hall, Boronic Acids: Preparation and Applications in Organic Synthesis Medicine and Materials, 2nd ed., Wiley-VCH, Weinheim 2011, pp. 427-477.
- (a) Kai Yang, Feng Zhang, Tongchang Fang, Guan Zhang and Qiuling Song*, Chem. Int. Ed. 2019, 58, 13421-13426. (b) Kai Yang, Xiaoxiao Hu, Wangyang Li, Jian Qiu, Qiang Feng, Shihui Wang, Guan Zhang, Zhijie Kuang, Peiyuan Yu* and Qiuling Song*, Cell Reports Physical Science, 2020, 1, 100268. (c) Kai Yang, Feng Zhang, Tongchang Fang, Chaokun Li, Wangyang Li, and Qiuling Song*, Nat. Commun. 2021, 12, 441.
|
 09:10 – 09:25
09:10 – 09:25 |
Invited Lecture Xin-Yuan Liu, Southern University of Science and Technology
Copper(I)-Catalyzed Radical-Involved Enantioconvergent Cross-Coupling Reactions
|
Copper(I)-Catalyzed Radical-Involved Enantioconvergent Cross-Coupling Reactions
Xin-Yuan Liu. Department of Chemistry, Southern University of Science and Technology, Shenzhen 518055, China. E-mail : liuxy3@sustech.edu.cn
Radical reactions have emerged as one of the most powerful and efficient tools for the construction of carbon-carbon and carbon-heteroatom bonds in organic synthesis. However, the development of catalytic asymmetric radical reactions to realize the stereochemical control of open-shell intermediates still remains a formidable challenge owing to the high reactivity of such free radical species. To solve this problem, our group has accomplished the radical-involved asymmetric chemistry by developing copper(I)/chiral anionic ligand as a novel single-electron-transfer (SET) catalyst. The key to success is the use of chiral reactive copper species to interact with the in-situ generated alkyl radicals to undergo either the formation of high-valent copper(III) complex followed by reductive elimination or a single-electron oxidation process to afford a carbocation intermediate, thereby resulting in a stereocontrol induction. In this talk, we will present copper-catalyzed radical-involved enantioconvergent cross-coupling reactions with our designed anionic chiral ligands.
|
 09:25 – 09:30
09:25 – 09:30 |
Interactive Break
|
|
 09:30 – 09:50
09:30 – 09:50 |
Keynote Lecture Guy Bertrand, University of California, San Diego
Carbenes as Powerful Transition-Metal Surrogates
|
Carbenes as Powerful Transition-Metal Surrogates
Guy Bertrand, University of California, San Diego. E-mail: gbertrand@ucsd.edu
It has been previously demonstrated that stable singlet electrophilic carbenes can behave as metal surrogates in the activation of small molecules and enthalpically strong E-H bonds, but it was believed that these activations only proceed through an irreversible activation barrier. We will show that, as it is the case with transition metals, the steric environment can be used to promote a reductive elimination at a carbon center.
Along this line, we will show that stable bicyclic (alkyl)(amino)carbenes allow for the stoichiometric carbonylation of ortho-quinones, the catalytic version being hampered by the reaction of the carbene with the quinones. However, the use of a bulky cyclic (alkyl)(amino)carbenes avoids this quenching, and thus allows for the catalytic carbonylation reaction into the corresponding cyclic carbonates.
H/D exchange at formyl groups is the most direct approach for the synthesis of deuterated aldehydes. Until now, only platinum-group metal complexes were known to catalyze this transformation, with significant substrate scope limitations. We have found that mesoionic carbenes catalyze the H/D exchange of aryl, alkenyl and alkyl aldehydes in high yields and deuterium incorporation levels using deuterated methanol as an affordable D source.
|
 09:50 – 10:05
09:50 – 10:05 |
Invited Lecture Bo Liu, Sichuan University
Synthetic Studies Toward Daphnane-type Diterpenoids and Crotophorbolone
|
Synthetic Studies Toward Daphnane-type Diterpenoids and Crotophorbolone
Bo Liu, Sichuan University. E-mail: chembliu@scu.edu.cn
Daphnane-type and tigliane-type diterpenoids are two big families of natural products with challenging 5/7/6 tricyclic skeleton decorated with 5-8 stereogenic centers. These natural compounds show impressive tumor-promoting, anti-cancer and anti-HIV bioactivities. Crotophorbolone is biosynthetically and structurally related to tigliane diterpenoids, and has been manifested by the Wender group transformable toward prostratin and DPP, two diterpenes exhibiting potent in vitro anti HIV activity. Several elegant total syntheses and numerous synthetic studies aiming at daphnane-type and tigliane-type diterpenoids have been reported since their isolation and structural elucidation. In this lecture, convergent synthetic strategy, starting from cheap and commercially available compound, toward daphnane-type diterpenoids and crotophorbolone will be discussed.
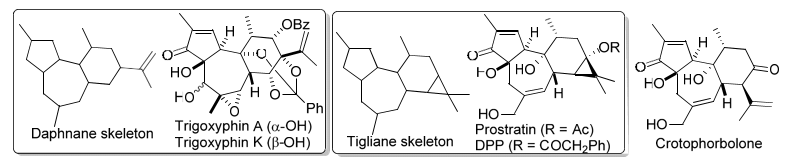
|
 10:05 – 10:35
10:05 – 10:35 |
Keynote Lecture Xuechen Li, The University of Hong Kong
Ten-Year Study on Daptomycin
|
Ten-Year Study on Daptomycin
Xuechen Li, Department of Chemistry, University of Hong Kong. E-mail: xuechenl@hku.hk
The existing pool of antibiotic-resistant bacterial pathogens has posed an ever-increasing threat to public health–according to a World Health Organization report in 2017, the antibiotic resistance threat is a current and global issue. New antibacterial drugs are urgently needed. However, owing to the scientific challenges and profit considerations, the process of new antibiotic development has been slow compared with other types of drugs. This situation calls for more efforts not only pharmaceutical companies but also academics researchers for iterative cycles of antibiotic discovery. In this regard, we have been working on the total synthesis and medicinal chemistry of some cyclic peptide-based antibiotics. Daptomycin was approved by the FDA in 2003 for the treatment of systemic and life-threatening infections caused by Gram-positive pathogens. It is a lipodepsipeptide and represents the newest first-in-class antibiotic developed over the past two decades. In 2013, we completed the first total synthesis of daptomycin, for which the Serine/Threonine Ligation (STL)-mediated peptide cyclization strategy was developed.1 Furthermore, we have conducted a medicinal chemistry study on daptomycin and established a comprehensive structure-active relationship,2 from which we discovered kynomycin as a new daptomycin analogue with improved antibacterial profile against both susceptible and resistant pathogens.3 In addition, we have developed a daptomycin-based probe to investigate the alternative bactericidal mechanism of daptomycin.4
References:
- Lam, H. Y.; Zhang, Y.; Liu, H.; Xu, J.; Wong, C. T.; Xu, C.; Li, X. J. Am. Chem. Soc. 2013, 135, 6272.
- Establishing the structure-activity relationship of daptomycin. Chow, H. Y.; Po, K. H. L.; Jin, K.; Qiao, G.; Sun, Z.; Ma, W.; Ye, X.; Zhou, N.; Chen, S.; Li, X. ACS Med. Chem. Lett. 2020, 11, 1442-1449.
- Chow, H. Y.; Po, K. H. L.; Gao, P.; Blasco, P.; Wang, X.; Li, C; Ye, L.; Jin, K.; Chen, K.; Chan, E. W. C.; You, X.; Kao, R. Y. T.; Chen, S.; Li, X. J. Med. Chem. 2020, 63, 3161.
- Blasco, P.; Zhang, C.; Chow, H. Y.; Chen, G.; Wu, Y.; Li, X. 2021 submitted
|
 14:00 – 16:20
14:00 – 16:20 |
Session 4 - Evening |
|
 14:00 – 14:20
14:00 – 14:20 |
Keynote Lecture Chihaya Adachi, Kyushu University
Exciton Management through CT Interaction in OLEDs and Organic Lasers
|
Exciton Management through CT Interaction in OLEDs and Organic Lasers
Chihaya Adachi, Kyushu University and the Center for Organic Photonics & Electronics Research. E-mail: adachi@cstf.kyushu-u.ac.jp
In the last three decades there have been extensive developments on organic luminescence materials aimed for high performance OLEDs. Sophisticated tuning of singlet, triplet, HOMO and LUMO energy levels provided a various kind of unique charge transfer states, enabling the creation of novel room temperature phosphorescence, thermally activated delayed fluorescence (TADF), long persistent luminescence (LPL) and so on. In this talk, we mention the future prospect of organic luminescence materials aiming for advanced devices such as organic semiconductor laser diodes (OSLDs).
|
 14:20 – 14:40
14:20 – 14:40 |
Keynote Lecture Qilong Shen, Shanghai Institute of Organic Chemistry
Fluoroalkyl-Substituted Sulfonium Ylides: A New Type of Electrophilic Fluoroalkylating Reagents
|
Fluoroalkyl-Substituted Sulfonium Ylides: A New Type of Electrophilic Fluoroalkylating Reagents
Qilong Shen, Shanghai Institute of Organic Chemistry, CAS. E-mail: shenql@sioc.ac.cn
Due to the well-known effect of the fluorine atom and fluorinated groups on the chemical, physical, and biological properties of a given molecule, incorporation of a fluorine or a fluoroalkyl group into compounds has become a routine practice in the development of drugs and agrochemicals. Consequently, development of efficient methods that could provide late-stage introduction of fluorine or fluorinated groups into bioactive compounds have been of intense interests.
Among the rapidly increasing and powerful fluoroalkylating methods, direct fluoroalkylation of a nucleophile with an electrophilic fluoroalkylating reagent arguably represents one of the most versatile and actively studied methods. Accordingly, several classes of electrophilic fluoroalkylating reagents has been developed by the groups of Yagupolskii, Umemoto, Togni, Shibata, and Prakash, among others, thus providing a strong driving force for the discovery of new fluoroalkylation methodologies. These reagents now allow the effective fluoroalkylation of a wide range of nucleophiles.
Even though some of these reagents have been commercialized, further broad applications of these electrophilic fluoroalkylating reagents has been hampered by their relatively complicated synthetic procedures. In the past three years, we have discovered a new family of electrophilic fluoroalkylating reagents—fluoroalkyl-substituted sulfonium ylides—that allow efficient fluoroalkylation of different nucleophiles under mild conditions. The reagents can be easily synthesized by a highly efficient rhodium-catalyzed carbenoid addition to a fluoroalkylthioether with a catalyst loading of 100 ppm-0.1 mol%. In addition, these new reagents can be readily scale-up and are stable to moisture and air. The low cost and structural flexibility of these compounds make them idea reagents for late-stage fluoroalkylation.
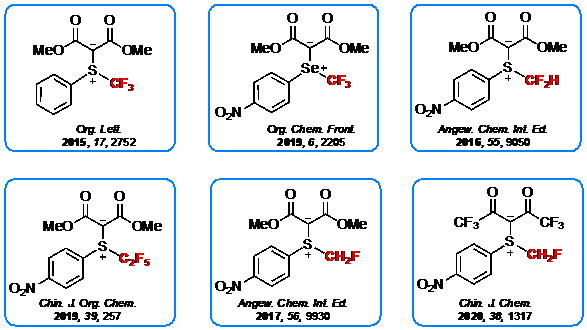
References:
- Liu, Y.-F.; Shao, X.-X.; Lu, L.; Shen, Q. Org. Lett. 2015, 17, 2752.
- Zhu, J.-S.; Liu, Y.-F. Shen, Q. Angew. Chem. Int. Ed. 2016, 55, 9050.
- Liu, Y.-F.; Lu, L.; Shen, Q. Chem. Int. Ed. 2017, 56, 9930.
- Ge, H.-M.; Shen, Q. Org. Chem. Front. 2019, 6, 2205.
- Ge, H.-M.; Wu, B.-T.; Liu, Y.-F.; Wang, H.-Y.; Shen, Q. ACS Cat. 2020, 10, 12414.
- Hong, X.; Liu, Y.-F.; Lu, L.; Shen, Q. Chin. J. Chem. 2020, 38, 1313.
|
 14:40 – 14:55
14:40 – 14:55 |
Invited Lecture Ho Yu Au-Yeung, The University of Hong Kong
Catalytic C–O Cross Coupling by Kinetically Stabilized, Catenane-Coordinated Copper Complexes
|
Catalytic C–O Cross Coupling by Kinetically Stabilized, Catenane-Coordinated Copper Complexes
Ho Yu Au-Yeung, Department of Chemistry, The University of Hong Kong. E-mail: hoyuay@hku.hk
Ligand design is fundamental to transition-metal catalyst development, and strategies such as varying the coordination donors and optimizing structural parameters of the ligand covalent framework are common directions in the search of better catalysts. Inspired by the high efficiency of metalloenzymes in which active metal sites are supported by peptide ligands, we propose that effective transition-metal catalysts could also be obtained by the use of mechanically bonded ligands that provide a shielded but assessable, kinetically stabilized metal center.
In this talk, a new C–O cross coupling of bromodicarbonyl and phenol that is catalyzed by catenane-supported copper(I) complexes will be discussed. Flexibility of the mechanical bond in the catenane ligand not only provides a responsive copper coordination for substrate transformation, but also allows the facile regeneration of the stable, coordinative saturated copper in the resting state. The catenane-supported copper catalyst is stable and the catalysis can be performed under air in high efficiency with a broad substrate scope.
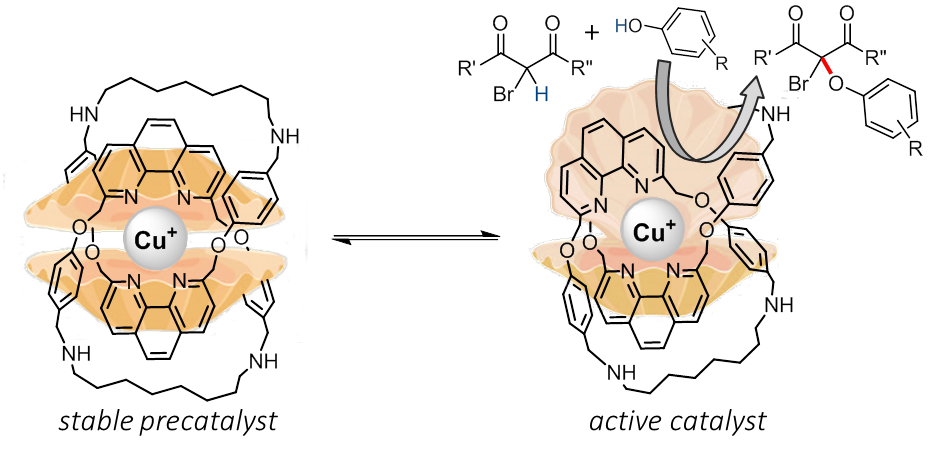 Figure 1. C–O cross coupling catalyzed by a catenane-coordinated copper(I) complex |
 14:55 – 15:00
14:55 – 15:00 |
Interactive Break
|
|
 15:00 – 15:20
15:00 – 15:20 |
Keynote Lecture Li-Zhu Wu, Technical Institute of Physics & Chemistry
Artificial Photosynthesis for Chemical Transformation
|
Artificial Photosynthesis for Chemical Transformation
Li-Zhu Wu, Technical Institute of Physics and Chemistry, CAS, Beijing 100190, China E-mail: lzwu@mail.ipc.ac.cn
Inspired by the ability of natural photosynthesis to convert solar energy into chemical energy, the scientific community long ago recognized the potential of light-driven reactions (photochemistry) as a powerful approach to chemical synthesis. From the high energy intermediate generated by photoinduced excitation of organic molecules, unique reaction manifolds can be accesses that are generally unavailable to conventional thermal pathways. Thus photochemical reactions considerably enrich the synthetic repertoire of modern organic chemists. Our group has long engaged in the photochemistry research related to the photoinduced energy transfer, electron transfer and chemical transformation. In this presentation, we will compile several stories to illustrate photochemical approaches that may be useful in the design of artificial photosynthetic systems for effective chemical transformation.
|
 15:20 – 15:40
15:20 – 15:40 |
Keynote Lecture Ching-Wen Chiu, National Taiwan University
Reactivity Studies of Boron Containing Radicals
|
Reactivity Studies of Boron Containing Radicals
Wei-Chun Liu, Ming-Han Chung, Isaac Furay Yu, Ching-Wen Chiu* Department of Chemistry, National Taiwan University, No. 1, Sec. 4, Roosevelt Rd., Taipei, Taiwan 10617. E-mail: cwchiu@ntu.edu.tw
Trivalent boron derivatives are promising electron transporting materials because of the intrinsic electron deficiency of boron. In the past few years, boron containing radicals and diradicals have been generated and even isolated. As an extension to our previous work on boryl functionalized phenoxyl radical,[1] we have prepared a series of boron-linked bis-phenol radicals and investigated the boron-mediated spin delocalization with EPR spectroscopy.[2] Our results suggested that the replacing of sp2 carbon center of a p-conjugate organic radical with a boron atom destabilizes the open-shell system due to the enhanced polarization of spin. In addition to the tri- coordinate boron radicals, we are also interested in the elusive di-coordinate neutral boron radical,[3] where the boron center bears merely five valence electrons. To overcome the electron deficiency of the di-substituted boron radical, strategies utilized in borinium cation and boryl radical were adopted. To this end, a di- coordinate boron radical cations stabilized by p-donating amino and p-accepting cAAC ligand were isolated and structurally characterized.[4] Preliminary reactivity studies of the new class boron radical will be discussed.
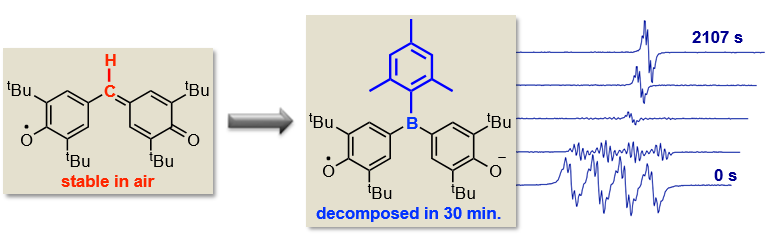 Figure 1: Destabilizing effect of boryl group on p-conjugate organic free radical.
References:
[1] Feng, P.-Y.; Liu, Y.-H.; Lin, T.-S.; Peng, S.-M.; Chiu, C.-W. Angew. Chem. Int. Ed., 2014, 53, 6237
[2] Chung, M.-H.; Yu, I. F.; Liu, Y.-H.; Lin, T.-S.; Peng, S.-M.; Chiu, C.-W. Inorg.
Chem., 2018, 57, 11732
[3] Liu, W.-C.; Liu, Y.-H.; Lin, T.-S.; Peng, S.-M.; Chiu, C.-W. Inorg. Chem., 2017, 56,10543
[4] Liu, W.-C.; Lin, Y.-C.; Tseng, H.-C.; Liu, Y.-H.; Chiu, C.-W. unpublished
|
 15:40 – 16:10
15:40 – 16:10 |
Plenary Lecture Makoto Fujita, The University of Tokyo
Coordination Self-Assembly: From the Origins to the Latest
Advances
|
Coordination Self-Assembly: From the Origins to the Latest Advances
Makoto Fujita. Department of Applied Chemistry, School of Engineering, The University of Tokyo, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: mfujita@appchem.t.u-tokyo.ac.jp
Molecular self-assembly based on coordination chemistry has made an explosive development in recent years. Over the last >25years, we have been showing that the simple combination of transition-metal’s square planer geometry (a 90 degree coordination angle) with pyridine-based bridging ligands gives rise to the quantitative self-assembly of nano-sized, discrete organic frameworks. Representative examples include square molecules (1990), linked-ring molecules (1994), cages (1995), capsules (1999), and tubes (2004) that are self-assembled from simple and small components. Originated from these earlier works, current interests in our group focus on i) molecular confinement effects in coordination cages, ii) solution chemistry in crystalline porous complexes (as applied to “crystalline sponge method”),[1] and iii) and giant self-assemblies[2] (Figure 1), as disclosed in this lecture.
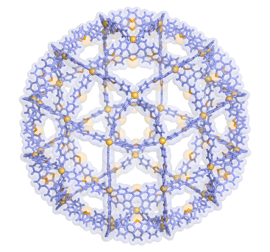 Figure 1. X-ray structure of M48L96 complex.
Reference
[1] Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, Y. Hitora, K. Takada, S. Matsunaga, K. Rissanen, M. Fujita Nature 2013, 495, 461-466.
[2] Fujita, Y. Ueda, S. Sato, N. Mizuno, T. Kumasaka, M. Fujita, Nature 2016, 540, 563.
|
 16:10 – 16:15
16:10 – 16:15 |
Poster Award Announcement |
|
 16:15 – 16:20
16:15 – 16:20 |
Closing Remarks - Day 2 |
|