|
|
Understanding biology by understanding chemistry | |
|
|
Registration Check-In; Attendees Seated and Poster Setup 13:00 - 14:30 | |
|
|
ACS Publications Introduction & Conference Overview | |
|
|
Keynote Lecture Shana J. Sturla, Professor of Toxicology, ETH Zürich "Tracking the Chemical Basis of Mutagenesis" |
Tracking the chemical basis of mutagenesis How can tools from chemical biology help us shift the early diagnosis paradigm in cancer to early risk prognosis? This is a question that motivates our research to understand mechanisms that govern reactions of mutagenic environmental chemicals with DNA, and characterize interactions of resulting DNA adducts with DNA damage and tolerance proteins and their networks in cells. Forecasting how these processes give rise to distinct mutational signatures in cancer necessitates strategies for chemical-specific mapping of modifications on a genome-wide level. The talk will explain new strategies we have developed for mapping chemical modifications in genomes, with a particular focus on toxicologically relevant DNA modification products arising from oxidation and alkylation reactions with nuclear DNA. Furthermore, we will address how mutations arise from error-prone translesion DNA synthesis catalyzed by specialized DNA polymerases, with an in-depth focus on how the chemical structures of nascent base pairs influence DNA synthesis rates, fidelities and ultimate mutagenic outcomes in cells.
Shana J. Sturla |
|
|
Keynote Lecture Florian Hollfelder, Professor in Chemical and Synthetic Biology, University of Cambridge "How Do We Find New Functional Proteins in Sequence Space?" |
How Do We Find New Functional Proteins in Sequence Space? Functional proteins for a variety of useful applications, as binders and catalysts, are required, but currently not known. Functional metagenomics and directed evolution promise access to such new proteins, but the chances of finding them are low. Therefore high-throughput technologies are crucial to beat the odds: screening in picoliter water-in–oil emulsion droplets produced in microfluidic devices allow screening of >10e7 clones and permit successful selections While potentially faster, the vastness of sequence space (and the scarcity of ‘solutions’ in it) require strategies for the identification and interconversion of enzymes. In this context the role of ‘promiscuous’ enzymes, sequencing of full length of genes at high throughput (UMIC-seq) and insertion/deletion mutagenesis (using the transposon-based method TRIAD) will be discussed. Together with a molecular and mechanistic understanding new routes to functional can be charted.
Florian Hollfelder
References: [1] Neun, S.; Brear, P.; Campbell, E.; Tryfona, T.; El Omari, K.; Wagner, A.; Dupree, P.; Hyvonen, M.; Hollfelder, F. Functional metagenomic screening identifies an unexpected beta-glucuronidase. Nat Chem Biol 2022, doi: 10.1038/s41589-022-01071-x |
|
|
Break |
Spotlighting the Functional Responsivity & Druggability of the Local Interactome in Living Systems I will highlight how an organic chemistry-driven idea has evolved into an enabling chemical biology toolset that can address some of the long-standing as well as emerging key biomedical problems of importance in both fundamental and translational science research. Focus will be placed on our latest understanding of precision electrophile signaling mechanisms and how such precision localized electrophile delivery concept and associated precision tools open up new opportunities to simultaneously map and functionally validate our localized protein players and pathways in a chemical-function-guided manner, in the context of functional drug discovery & medicinal chemistry.
Yimon Aye |
|
|
Keynote Lecture Yimon Aye, Professor of Chemistry, EPFL "Spotlighting the Functional Responsivity & Druggability of the Local Interactome in Living Systems" |
Spotlighting the Functional Responsivity & Druggability of the Local Interactome in Living Systems I will highlight how an organic chemistry-driven idea has evolved into an enabling chemical biology toolset that can address some of the long-standing as well as emerging key biomedical problems of importance in both fundamental and translational science research. Focus will be placed on our latest understanding of precision electrophile signaling mechanisms and how such precision localized electrophile delivery concept and associated precision tools open up new opportunities to simultaneously map and functionally validate our localized protein players and pathways in a chemical-function-guided manner, in the context of functional drug discovery & medicinal chemistry.
Yimon Aye |
|
|
Keynote Lecture Stuart Conway, Professor of Organic Chemistry, University of Oxford "Probing the Function of Bromodomains in Human Disease" |
Probing the Function of Bromodomains in Human Disease Tumor hypoxia (low oxygen) is associated with therapy resistance and poor patient prognosis. Hypoxia-activated prodrugs, which target oxygen-deficient cells, represent a promising treatment strategy. We have demonstrated the pre-clinical efficacy of NI-Pano, a novel hypoxia-activated pro-drug of the clinically used lysine deacetylase inhibitor, panobinostat. NI-Pano is stable in normoxic (21% oxygen) conditions and undergoes NADPH-CYP-mediated enzymatic bioreduction to release panobinostat in hypoxia (<0.1% oxygen). NI-Pano exhibited growth delay effects as a single agent in mouse tumor xenografts. Pharmacokinetic analysis confirmed the presence of panobinostat in hypoxic mouse xenografts, but not in circulating plasma or kidneys. Our preclinical results provide a strong mechanistic rationale for the clinical development of NI-Pano for selective targeting of hypoxic tumors. Work to develop complementary imaging agents for hypoxia will also be discussed.
Prof. Stuart Conway |
|
|
Closing Remarks - Day 1 | |
|
|
Drug discovery, design and delivery: PART 1 | |
|
|
ACS Publications Welcome & Introduction | |
|
|
Keynote Lecture Kelly Chibale, Professor of Organic Chemistry, University of Cape Town "Medicinal Chemistry and Chemical Biology at the H3D Centre" |
Medicinal Chemistry and Chemical Biology at the H3D Centre Bridging the gap between basic science and clinical research to advance innovative medicines discovery requires, amongst other things, the integration of medicinal chemistry with biology and preclinical pharmacology, including drug metabolism and pharmacokinetics studies. This talk will introduce the University of Cape Town (UCT) Holistic Drug Discovery and Development Centre (H3D). It will also describe the establishment of chemistry, biology and pharmacology platforms and how they have been deployed in drug discovery projects underpinned by medicinal chemistry and chemical biology.
Kelly Chibale
Kelly Chibale1,2 1Holistic Drug Discovery and Development (H3D) Centre, University of Cape Town, Rondebosch 7701, South Africa References: [1] Winks et al Nature Medicine, 2022, https://doi.org/10.1038/s41591-022-01885-1 |
|
|
Keynote Lecture Stefan Laufer, Professor of Pharmaceutical Chemistry, University of Tübingen "Targeting the R-Spine: Design, Synthesis and Biological Evaluation of Novel Type I½ p38α MAP Kinase Inhibitors with Excellent Selectivity, High Potency and Prolonged Target Residence Time " |
Targeting the R-Spine: Design, Synthesis and Biological Evaluation of Novel Type I½ p38α MAP Kinase Inhibitors with Excellent Selectivity, High Potency and Prolonged Target Residence Time
Michael Forster1,2 , Stefan Laufer1,2,3 1IFIT Cluster of Excellence EXC 2180 ‘Image-Guided and Functionally Instructed Tumor Therapies’, University of Tuebingen, Tuebingen, Germany. 3Tübingen Center for Academic Drug Discovery & Development (TüCAD2), Tuebingen, Germany p38 MAP kinase inhibitors are widely investigated for a plethora of inflammatory diseases including RA and COPD. Latest results, however, may open an avenue for cancer and CNS-diseases as well. Although for such indications, inhibitors with very particular properties are necessary. We recently reported Skepinone-L as a Type I p38α MAP kinase inhibitor with high potency and excellent selectivity in vitro and in vivo.[1] However, as a Type I inhibitor, it acts entirely ATP-competitive and shows just a moderate residence time. Thus, the scope was to develop a new class of advanced compounds maintaining the structural binding features of Skepinone-L scaffold like inducing a glycine flip at the hinge region and occupying both hydrophobic regions I and II. Extending this scaffold with suitable residues resulted in an interference with the kinase’s R-Spine. By optimizing this interaction, we could significantly prolong the target residence time up to 4.000 s, along with an excellent selectivity-score of 0.006 and an outstanding subnanomolar potency. This new binding mode was validated by co-crystallization, showing all binding interactions typifying Type I½ binding [2,3]. Long term MD-simulation underlines the decisive role of water and protein dynamics in residence time of p38a MAP kinase inhibitors [4]. Based on the concept of Type I½ inhibitors with improved TRT, the drug development project Improve-CRC funded by the Landesstiftung Baden-Württemberg was initiated in 2018. [5] A comprehensive screening platform was established including enzymatic and cellular assays as well as TRT determinations and patient-derived organoid assays. The characterization of a library containing more than 300 potential Type I½ binders combined with in silico methods and rational design for the ongoing synthesis of new derivatives finally led to the identification of a preclinical candidate to treat colorectal cancer, which underwent extensive in vitro and in vivo characterization. In parallel, the synthetic access of key intermediates underwent an extensive optimization to ensure the sufficient supply of the emerging pre-clinical development. [6]
References:
Stefan Laufer |
|
|
Keynote Lecture Olivia M. Merkel, Professor of Drug Delivery, LMU Munich "Hydrophobic Modification of Spermine-based Poly(β-amino ester)s and its Role in siRNA Delivery" |
Hydrophobic modification of spermine-based poly(β-amino ester)s and its role in siRNA delivery After RNAi was first discovered over 20 years ago, siRNA-based therapeutics are finally becoming reality. However, the delivery of siRNA has remained a challenge. In our previous research, we found that the spermine-based poly(β-amino ester)s are very promising for siRNA delivery. However, the role of hydrophobic modification in siRNA delivery of spermine-based poly(β-amino ester)s is not fully understood yet. In this research, we synthesized spermine-based poly(β-amino ester)s composed of different percentages of oleylamine side chains, named P(SpOABAE). The chemical structures of the polymers were characterized by 1H NMR. The polymers showed efficient siRNA encapsulation determined by SYBR gold assays. The hydrodynamic diameters of the P(SpOABAE) polyplexes from N/P 1 to 20 were 30 – 100 nm except for aggregation phenomena observed at N/P 3. The cellular uptake of the polyplexes was determined by flow cytometry in H1299 cells, and all the polyplexes showed significantly higher cellular uptake than hyperbranched polyethylenimine (hyPEI, 25 kDa). The P(SpOABAE) polyplexes were able to achieve more than 90% GFP knockdown in H1299/eGFP cells and Sp0.7/OA0.3 polyplexes at N/P 5 mediated a similar GAPDH knockdown as the commercial transfection reagent LipofectamineTM 2000 in 16HBE14o- cells. Most importantly, in contrast to many other PBAEs, the materials described here encapsulate and deliver siRNA at a very low polymer excess rate, reducing concerns of polycation-mediated toxicity. 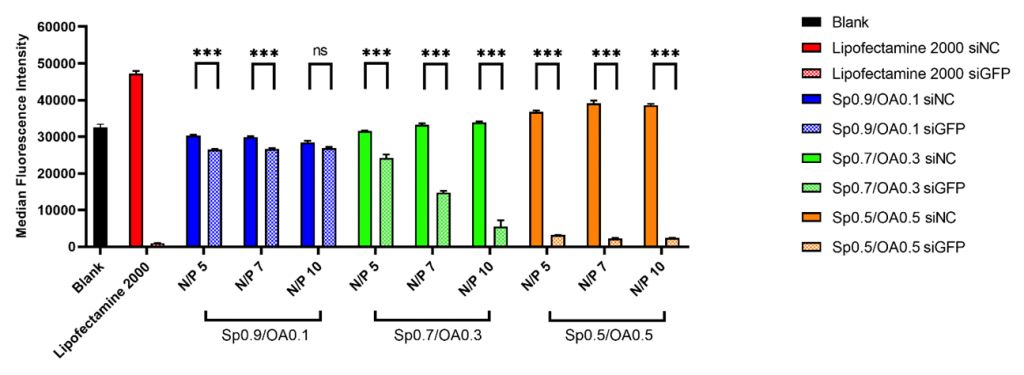 Figure 1. Enhanced green fluorescent protein (eGFP) knockdown efficiency in H1299-eGFP cells after transfection with Sp0.9/OA0.1, Sp0.7/OA0.3, and Sp0.5/OA0.5 polyplexes at N/P 5, 7 and 10 for 48 h. Positive control: LipofectamineTM 2000 formulated. Average ± SD, n = 3, One-way ANOVA with Bonferroni post-hoc test, nsp > 0.05, *** p < 0.001.
Olivia Merkel https://www.cup.lmu.de/pb/aks/merkel/ |
|
|
Break | |
|
|
Keynote Lecture Hans Lennernäs, Professor of Biopharmacy, Uppsala University "Image-guided locoregional precision dosing of chemotherapeutics in liver cancer and supportive treatment of their off-target effects in the small intestine" |
Image-guided locoregional precision dosing of chemotherapeutics in liver cancer and supportive treatment of their off-target effects in the small intestine Hepatocellular carcinoma (HCC) is a common cause of cancer-related death, often detected in the intermediate stage. The standard of care for intermediate-stage HCC is transarterial chemoembolisation (TACE), where idarubicin (IDA) is a promising drug. Despite the fact that TACE has been used for several decades, treatment success is unpredictable. We have an on-going Phase II clinical trial that has been designed to further improve TACE by increasing the understanding of interactions between local pharmacology, tumor targeting, HCC pathophysiology, metabolomics and molecular mechanisms of drug resistance. In addition, to improve the effect of any cancer treatment it is important to have an in-depth understanding of drug diffusion and, penetration across tumor extracellular matrix and cellular uptake. In one of our project, we have developed a miniaturized chip where drug gradient formation and cellular uptake in different hydrogel environments can be investigated quantified at high resolution using live imaging. These data will be compared with data from our preclinical models and human patients of how to optimize locoregional treatments of cancer. Doxorubicin and idarubicin, two anthracyclines, induce cytotoxicity in tumor cells and off-target effect on the gastrointestinal system. To investigate the chemotherapeutic intestinal mucositis (CIM) rats were dosed with doxorubicin intravenously (iv). The main effect parameter was intestinal villus atrophy and was most severe after three days following a single dose of doxorubicin. This was preceded by an increased cell death and reduced proliferation in the intestinal crypts, which occurred within the first day after doxorubicin dosing. We also examined how six different cytostatic drugs iv affected chemotherapy-induced mucositis in rats, and to what extent they caused diarrhea. All six cancer drugs caused similar intestinal villus atrophy three days after single iv dosing, and idarubicin and irinotecan caused clear diarrhea. In other pre-clinical project our aim was to develop supportive treatments and prevention for idarubicin-induced intestinal mucositis and diarrhea, by iv dosing of anakinra and/or dexamethasone (anti-inflammatory effects). Anakinra alone stopped the diarrhea, while the combination of anakinra and dexamethasone prevented intestinal villus atrophy. These positive effects encourage further investigations into the clinical use of anakinra and dexamethasone in optimized drug delivery systems as supportive therapies for chemotherapy-induced mucositis and diarrhea in patients.
Hans Lennernäs |
|
|
Keynote Lecture Dean Naisbitt, Professor of Drug Safety Science, The University of Liverpool "Deciphering Mechanisms of Adverse Drug Reactions" |
Deciphering mechanisms of adverse drug reactions The discovery of strong associations between expression of HLA alleles of MHC and specific forms of drug-induced immune-mediated tissue injury has changed the way in which researchers define this form of iatrogenic disease: reactions are no longer completely unpredictable. Studies with patient PBMC and PBMC from drug-naive donors carrying HLA risk alleles have shown that drugs form specific associations with HLA-risk alleles generating T-cell antigens and hence provide the immunogenetic basis for the reaction. Herein, I will describe our recent studies defining the chemical, cellular and genetic basis for different forms of drug-induced skin and liver injury. I will also address the enigma of immunological tolerance and explore the role tolerance plays in determination of susceptibility to such adverse events even in individuals carrying genetic and immunogenic liabilities.
Dean Naisbitt Twitter: @ImmunoPharm
|
|
|
Networking Lunch and Poster Session |
Metal-Ligand Cooperative Transformations of Small Molecules Yunho Lee, Seoul National University, Email: yunhochem@snu.ac.kr Metal-ligand cooperation (MLC) is currently receiving much attention as a novel synthetic methodology to expand the role of transition metals in organometallic reactions. The ligand in MLC delivers necessary electron, proton and/or a functional group to assist metal. While noninnocent phosphine ligands are relatively rare, our group recently reported a unique MLC by employing an anionic PPP ligand (PPP– = –P[2-PiPr2-C6H4]2). In this system, the reversible phosphide/phosphinite interconversion of a PPP ligand is coupled with a 2-electron redox change of nickel. Interestingly, the central phosphide moiety can act as a single electron donor, which was recognized from the studies of analogous nickel amide species. In this presentation, the MLC redox chemistry of a (PPP)M scaffold (M = Ni or Co) and its application in small molecule transformation will be presented. Both nickel and cobalt complexes reveal the reversible formation of a P–P bond involving a single electron exchange between metal and P. A P–P bond containing dimer, (P2P-PP2){Ni(CO)}2, shows radical-type reactivity toward various substrates under visible-light conditions. Detection of a metal-phosphinyl radical species formed upon irradiation was established by EPR spectroscopy and through experiments employing radical scavengers. The reactions with a phenoxyl radical clearly show the difference in reactivity of close-shell nickel and open-shell cobalt species.
|
|
|
Drug discovery, design and delivery: PART 2 |
Therapeutic Opportunities in Glycoscience Carolyn R. Bertozzi, Anne T. and Robert M. Bass Professor of Chemistry and Professor of Chemical & Systems Biology and Radiology (by courtesy), Stanford University; Baker Family Director, Stanford ChEM-H; Howard Hughes Medical Institute. E-mail: bertozzi@stanford.edu
Cell surface glycans constitute a rich biomolecular dataset that drives both normal and pathological processes. Their “readers” are glycan-binding receptors that can engage in cell-cell interactions and cell signaling. Our research focuses on mechanistic studies of glycan/receptor biology and applications of this knowledge to new therapeutic strategies. Our recent efforts center on pathogenic glycans in the tumor microenvironment and new therapeutic modalities based on the concept of targeted degradation. |
|
|
Keynote Lecture Felix Calderon, Tres Cantos Open Lab Head, GSK "From Open Innovation (OI) to Innovation in Partnership (IIP)" |
From Open Innovation (OI) to Innovation in Partnership (IIP) The last decade has witnessed the birth of a wide range of partnership models between academic institutions and pharmaceutical companies. The adoption of the Open Innovation principles for data-sharing in industry and the boost of the entrepreneurial spirit of academic scientist, has opened a countless number of collaborative initiatives hard to imagine 15 years ago. This change has been accompanied with multiple cultural and logistic challenges, but the benefits of overcoming them have become evident during the COVID-19 crisis. Endemic Infectious Diseases constitute a particular cluster of diseases where some of the most extremes models have been developed. The urgency for action and the opportunity for innovation are factors that have facilitated the creation innovative models. In 2010, GSK launched the Tres Cantos Open Lab, a collaborative test bed to boost innovation in drug discovery for Endemic Infectious Diseases. Governed by an independent board of recognized scientist in the field of Infectious Diseases; the Open Lab goes a step beyond the open sharing principles of Open Innovation or virtual collaboration, to offer a space where scientist from Academia and GSK can work together in the same projects. During these years, more than 90 projects have been implemented, delivering different kind of innovations. In this presentation we will analyse the opportunities that the OL has brought to the community and the field.
Felix Calderon |
|
|
Keynote Lecture Maria-Laura Bolognesi, Professor of Medicinal Chemistry, Alma Mater Studiorum - University of Bologna "Bifunctional Small Molecules in Medicinal Chemistry: When Two Are Better Than One" |
Bifunctional small molecules in medicinal chemistry: When Two Are Better Than One Bifunctional/bivalent small molecules resulting from the combination of two bioactive frameworks, are gaining a new momentum in medicinal chemistry. Molecular hybridization is a rational strategy that allows to combine two starting pharmacophores in a new, single bifunctional entity, able to modulate two targets of interest. Although promising for contrasting the multifactorial nature of neurodegenerative diseases,1 the development of such hybrids faces the critical issues of selecting the right target combination and achieving a balanced activity towards them, while maintaining drug-like-properties.2, 3 Similar concepts have inspired the design of bivalent protein-protein interaction inhibitors in Alzheimer’s disease. Considering the oligomeric and repetitive structure of fibrillar aggregates, bivalent compounds have been suggested to interact simultaneously with two binding surfaces. Thus, they could cross-link fibrils, and perturb the aggregation process.4 Recent development of PROteolysis TArgeting Chimeras (PROTACs) further emphasizes the potential of bifunctional ligands that bind to target proteins, but not necessarily inhibit their functions. In this talk, selected examples of bivalent small molecules from our own research will allow to discuss issues and consequences relevant to medicinal chemistry.5
Maria-Laura Bolognesi https://www.unibo.it/sitoweb/marialaura.bolognesi/en References: [1] Cavalli, A.; Bolognesi, M. L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. J Med Chem 2008, 51, 347-72.
|
|
|
Break |
Targets for broad-spectrum antiviral therapy: lessons learned and future perspectives The identification of compounds endowed with broad-spectrum mechanism of action is the key to limit the spread of emerging viruses. In the last years, globalization, global warming, and population aging have contributed to the spread of emerging viruses, such as Coronaviruses (COVs), West Nile (WNV), Dengue (DENV) and Zika (ZIKV). The number of reported infections is increasing and considering the high viral mutation rate it is conceivable that it will increase significantly in the next years. The risk caused by viruses is now more evident due to the COVID-19 pandemic, which highlighted the need to find new broad-spectrum antivirals, able to tackle the present pandemic and future epidemics. Direct-acting antivirals (DAAs) have been successfully developed to fight specific infections like HIV-1 and HCV. Nevertheless, targeting viral components shared among multiple viruses such as envelope and nonstructural proteins can represent a successful strategy to develop broad-spectrum antivirals. Viral infections cause the reprogramming of the cell machinery, which is used by the pathogen as a sort of viral factory, causing overexpression of many host’s proteins, in particular DDX3X. In this context, our group developed both direct and indirect-acting broad-spectrum antivirals, targeting envelope, NS5 methyl transferase and h-DDX3X protein 1-5. Our compounds are effective against persistent infections such as HIV, HCV and Flu, but also emerging viruses, such as the arboviruses DENV, WNV, JEV, ZIKV and SARS-CoV-2. The absence of in vivo toxicity and the activity against drug-resistant strains suggest that our inhibitors can represent safe and promising classes of antivirals, supporting their use alone or in combination with other drugs in the treatment of co-infections and emerging viruses.
Annalaura Brai https://en.unisi.it/ References: [1[ Brai, A.; Fazi, R.; Tintori, C.; Zamperini, C.; Bugli, F.; Sanguinetti, M.; Stigliano, E.; Esté, J.; Badia, R.; Franco, S.; et al. Human DDX3 Protein Is a Valuable Target to Develop Broad Spectrum Antiviral Agents. Proc. Natl. Acad. Sci. U. S. A. 2016, 113 (19), 5388–5393. |
|
|
Keynote Lecture Annalaura Brai, Senior Scientist, University of Siena "Targets for Broad-Spectrum Antiviral Therapy: Lessons Learned and Future Perspectives" |
Targets for broad-spectrum antiviral therapy: lessons learned and future perspectives The identification of compounds endowed with broad-spectrum mechanism of action is the key to limit the spread of emerging viruses. In the last years, globalization, global warming, and population aging have contributed to the spread of emerging viruses, such as Coronaviruses (COVs), West Nile (WNV), Dengue (DENV) and Zika (ZIKV). The number of reported infections is increasing and considering the high viral mutation rate it is conceivable that it will increase significantly in the next years. The risk caused by viruses is now more evident due to the COVID-19 pandemic, which highlighted the need to find new broad-spectrum antivirals, able to tackle the present pandemic and future epidemics. Direct-acting antivirals (DAAs) have been successfully developed to fight specific infections like HIV-1 and HCV. Nevertheless, targeting viral components shared among multiple viruses such as envelope and nonstructural proteins can represent a successful strategy to develop broad-spectrum antivirals. Viral infections cause the reprogramming of the cell machinery, which is used by the pathogen as a sort of viral factory, causing overexpression of many host’s proteins, in particular DDX3X. In this context, our group developed both direct and indirect-acting broad-spectrum antivirals, targeting envelope, NS5 methyl transferase and h-DDX3X protein 1-5. Our compounds are effective against persistent infections such as HIV, HCV and Flu, but also emerging viruses, such as the arboviruses DENV, WNV, JEV, ZIKV and SARS-CoV-2. The absence of in vivo toxicity and the activity against drug-resistant strains suggest that our inhibitors can represent safe and promising classes of antivirals, supporting their use alone or in combination with other drugs in the treatment of co-infections and emerging viruses.
Annalaura Brai https://en.unisi.it/ References: [1[ Brai, A.; Fazi, R.; Tintori, C.; Zamperini, C.; Bugli, F.; Sanguinetti, M.; Stigliano, E.; Esté, J.; Badia, R.; Franco, S.; et al. Human DDX3 Protein Is a Valuable Target to Develop Broad Spectrum Antiviral Agents. Proc. Natl. Acad. Sci. U. S. A. 2016, 113 (19), 5388–5393. |
|
|
Keynote Lecture Sarah E. O'Connor, Director, Max Planck Institute for Chemical Ecology "Harnessing the chemistry of plant natural product biosynthesis" |
Harnessing the chemistry of plant natural product biosynthesis Plants, which make thousands of complex natural products, are outstanding chemists. Through the concerted action of enzymes that are assembled into metabolic pathways, nature creates enormous chemical complexity from simple starting materials. This talk will highlight the discovery process for enzymes that catalyze unusual or unprecedented enzymatic transformations, mechanistic and structural characterization of these enzymes, and methods by which these enzymes can be harnessed for metabolic engineering to generate pharmacological important compounds. A variety of different plants and molecules are used for these studies, most notably the monoterpene indole alkaloids and the monoterpenes known as iridoids.
Sarah O’Connor https://www.sarahoconnor.org/
|
|
|
ACS Publications Session Wrap-up | |
|
|
Networking Reception | |
|
|
Engineering biology | |
|
|
ACS Publications Welcome & Introduction | |
|
|
Keynote Lecture Geoffrey Otim, Founder & CEO, SynBio Africa "The Status of Synthetic Biology and Biosecurity in Africa – The Past, Present and Future" |
The status of synthetic biology and biosecurity in Africa – the past, present and future. Aside from SynBio Africa, there are other organizations in the region’s synthetic biology ecosystem. These include Open Plant, Joint BioEnergy Institute, Biomarker Africa, Amino Labs, and the African Institute of Open Science and Hardware. These organizations provide services in open-source registries for plants and grants to start-up synthetic biology labs, hold pieces of training in synthetic biology laboratory techniques, supply cheap synthetic biology toolkits, and contribute a forum for key synthetic biology players to collaborate. Though regulatory policies for synthetic biology have yet to be developed in most African countries, some nations are already using certain provisions contained in genetically modified organism (GMO) regulations. In addition, Mudziwapasi et al (2022) reviewed recent progress in the agricultural applications of synthetic biology within the African context. For instance, the authors discussed how synthetic biology could address food insecurity by aiding the development of novel food products like nutraceuticals and probiotics and enhancing animal productivity. Moreover, synthetic biology could reduce fertilizer reliance, as well as develop local crops with desirable characteristics such as high yield, drought resistance, and pesticide resistance. The authors also highlighted the risks and disadvantages of synthetic biology in Africa, including the biopiracy of African resources, the production of certain crops at the expense of other crops, and the accidental release of engineered microorganisms into the environment.
Geoffrey Otim https://synbioafrica.com
|
|
|
Keynote Lecture Marnix Medema, Professor, Wageningen University & Research "Deciphering the Chemical Language of the Microbiome" |
Deciphering the chemical language of the microbiome using computational genomics An important challenge in microbiome science is to obtain an understanding of the mechanistic basis for many microbe-associated phenotypes. Microbial specialized metabolites are important mediators of molecular interactions between microbes as well as with the host, and in a way constitute the ‘chemical language’ of the microbiome. Hence, they are of great importance from both ecological and clinical perspectives. A range of computational methods have been developed to identify these molecules and the metabolic gene clusters that encode their production, and to assess their biological activities. Here, I will highlight recent work performed in my research group on developing and applying these approaches to accelerate natural product discovery, as well as to study the roles of these pathways in microbe-microbe and host-microbe interactions in microbiomes.
Marnix H. Medema http://www.marnixmedema.nl/ |
|
|
Break |
Deciphering the chemical language of the microbiome using computational genomics An important challenge in microbiome science is to obtain an understanding of the mechanistic basis for many microbe-associated phenotypes. Microbial specialized metabolites are important mediators of molecular interactions between microbes as well as with the host, and in a way constitute the ‘chemical language’ of the microbiome. Hence, they are of great importance from both ecological and clinical perspectives. A range of computational methods have been developed to identify these molecules and the metabolic gene clusters that encode their production, and to assess their biological activities. Here, I will highlight recent work performed in my research group on developing and applying these approaches to accelerate natural product discovery, as well as to study the roles of these pathways in microbe-microbe and host-microbe interactions in microbiomes.
Marnix H. Medema http://www.marnixmedema.nl/ |
|
|
Keynote Lecture Tobias Erb, Max Planck Society "Photosynthesis 2.0: Re-inventing CO2-fixation with Synthetic Biology and Machine Learning" |
Photosynthesis 2.0: Re-inventing CO2-fixation with Synthetic Biology and Machine Learning Synthetic biology offers exciting opportunities to realize new-to-nature functions that evolution has not invented, yet. In my talk, I will present efforts from our lab to develop novel CO2-fixing enzymes, pathways and artificial chloroplasts that are more efficient than natural photosynthesis. I will highlight the role of machine learning in this process and present new ways of how to power such biological systems with electricity in the future.
Tobias J. Erb
|
|
|
Keynote Lecture Birte Höcker, Biochemistry Professor, Bayreuth University "On the Design of Protein Folds and Functions" |
On the Design of Protein Folds and Functions Protein design aims to build new proteins with novel functions. Methods range from generating and screening mutant libraries via repurposing of active sites or binding pockets all the way to de novo design. In many of these approaches protein structures form the basis for the designs and structures are solved to validate the hypotheses. I will discuss advantages and difficulties of the different approaches and show some highlights from our recent applications that range from the repurposing of a repressor to a plant sensor via evolution-guided design by chimeragenesis to the de novo design of TIM-barrel proteins. As the rapidly developing AI-based methods also have a great potential for protein design, I will also touch on the use of natural language processing for protein design.
Birte Höcker |
|
|
Lunch and Poster Session |
On the Design of Protein Folds and Functions Protein design aims to build new proteins with novel functions. Methods range from generating and screening mutant libraries via repurposing of active sites or binding pockets all the way to de novo design. In many of these approaches protein structures form the basis for the designs and structures are solved to validate the hypotheses. I will discuss advantages and difficulties of the different approaches and show some highlights from our recent applications that range from the repurposing of a repressor to a plant sensor via evolution-guided design by chimeragenesis to the de novo design of TIM-barrel proteins. As the rapidly developing AI-based methods also have a great potential for protein design, I will also touch on the use of natural language processing for protein design.
Birte Höcker |
|
|
New tools |
Synthetic Studies Toward Daphnane-type Diterpenoids and Crotophorbolone Bo Liu, Sichuan University. E-mail: chembliu@scu.edu.cn
Daphnane-type and tigliane-type diterpenoids are two big families of natural products with challenging 5/7/6 tricyclic skeleton decorated with 5-8 stereogenic centers. These natural compounds show impressive tumor-promoting, anti-cancer and anti-HIV bioactivities. Crotophorbolone is biosynthetically and structurally related to tigliane diterpenoids, and has been manifested by the Wender group transformable toward prostratin and DPP, two diterpenes exhibiting potent in vitro anti HIV activity. Several elegant total syntheses and numerous synthetic studies aiming at daphnane-type and tigliane-type diterpenoids have been reported since their isolation and structural elucidation. In this lecture, convergent synthetic strategy, starting from cheap and commercially available compound, toward daphnane-type diterpenoids and crotophorbolone will be discussed. |
|
|
Keynote Lecture Gonçalo Bernardes, Professor of Chemical Biology, University of Cambridge "Translational Chemical Biology" |
Translational Chemical Biology Our research uses chemistry principles to address questions of importance in life sciences and molecular medicine. This lecture will cover recent examples of emerging areas in our group in:
Gonçalo J. L. Bernardes |
|
|
Keynote Lecture Magnus Palmblad, Associate Professor, Leiden University Medical Center "Semantic Annotation and Text Mining of Analytical Chemistry Methods" |
Semantic Annotation and Text Mining of Analytical Chemistry Methods The who, when, where, what, why and how are a set of simple but effective questions one can ask whenever approaching a new topic or analyzing a body of scientific literature. Who published the research, when, and where are relatively straightforward to answer, even if author disambiguation can be a major challenge. Steady advances in machine learning, text mining and computational chemistry now enable us to also answer specific what, how and even why questions directly from the literature. What types of samples. compounds or chemical reactions have been studied, and how? What are the relative strengths of different analytical chemistry methods? In this talk, I will describe some free and open source tools that can be used to automate these analyses and create interactive visualizations summarizing thousands of papers. Extraction of more complex and formal method metadata from natural language descriptions is a hard problem. I will describe some of our recent efforts in abstraction of experimental methods in analytical chemistry. These formal abstractions that may be used to validate machine learning methods for extracting method metadata from experimental sections in scientific papers. They can also be used to compare and analyze the methods themselves.
Magnus Palmblad |
|
|
Keynote Lecture Christa Müller "Developing modulators of purinergic signaling to target inflammation and cancer" |
Developing modulators of purinergic signaling to target inflammation and cancer PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical & Medicinal Chemistry, University of Bonn, An der Immenburg 4, 53121 Bonn, Germany
Extracellular adenosine triphosphate (ATP) acts as a pro-inflammatory danger signal via activation of purine P2Y and P2X receptors. In contrast, its corresponding nucleoside adenosine is a strongly immunosuppressive agent activating G protein-coupled P1 (adenosine) receptors. Cancer tissues can release large amounts of the nucleotide ATP, which is immediately hydrolyzed by ectonucleotidases, that are upregulated on many cancer cells, leading to the production of adenosine. Activation of adenosine A2A and A2B receptors results in antiproliferative, angiogenic, pro-metastatic, and strongly immunosuppressive effects. The balance between the impact of pro-inflammatory ATP and anti-inflammatory adenosine can be modulated by ectonucleotidase inhibitors, or by activation or blockade of purine receptor subtypes. Recent progress of our laboratory in the identification and optimization of purine receptor antagonists and ectonucleotidase inhibitors by convergent approaches, utilizing structural biology, will be presented. These tool compounds, including labeled derivatives, are used to study their targets’ role in health and disease. Moreover, they have potential for further development as novel drugs.
Christa Müller http://mueller-group.pharma.uni-bonn.de |
|
|
Closing Remarks & Poster Winners |
Developing modulators of purinergic signaling to target inflammation and cancer PharmaCenter Bonn, Pharmaceutical Institute, Pharmaceutical & Medicinal Chemistry, University of Bonn, An der Immenburg 4, 53121 Bonn, Germany
Extracellular adenosine triphosphate (ATP) acts as a pro-inflammatory danger signal via activation of purine P2Y and P2X receptors. In contrast, its corresponding nucleoside adenosine is a strongly immunosuppressive agent activating G protein-coupled P1 (adenosine) receptors. Cancer tissues can release large amounts of the nucleotide ATP, which is immediately hydrolyzed by ectonucleotidases, that are upregulated on many cancer cells, leading to the production of adenosine. Activation of adenosine A2A and A2B receptors results in antiproliferative, angiogenic, pro-metastatic, and strongly immunosuppressive effects. The balance between the impact of pro-inflammatory ATP and anti-inflammatory adenosine can be modulated by ectonucleotidase inhibitors, or by activation or blockade of purine receptor subtypes. Recent progress of our laboratory in the identification and optimization of purine receptor antagonists and ectonucleotidase inhibitors by convergent approaches, utilizing structural biology, will be presented. These tool compounds, including labeled derivatives, are used to study their targets’ role in health and disease. Moreover, they have potential for further development as novel drugs.
Christa Müller http://mueller-group.pharma.uni-bonn.de |
